'Gene desert' regulates embryonic development and cardiac function
Researchers at the University of Bern, in collaboration with international partners, have discovered that a ‘gene desert’ section of the genome plays an important role in the development of the embryo and the heart in both mice and humans. The study provides further evidence for the significance of gene-free DNA segments in gene regulation and offers approaches for early detection of cardiac diseases.
The human genome contains specific sections of DNA, known as ‘genes’, which serve as blueprints for proteins. When genes are altered as a result of so-called ‘mutations’, this can lead to defective proteins, resulting in disease or malformations of the embryo. Genome research has shown that mutations can also cause problems in DNA regions that do not carry a gene. Such regions, often mistakenly referred to as ‘junk DNA’ can play a crucial role in the regulation of gene expression and embryonic development.
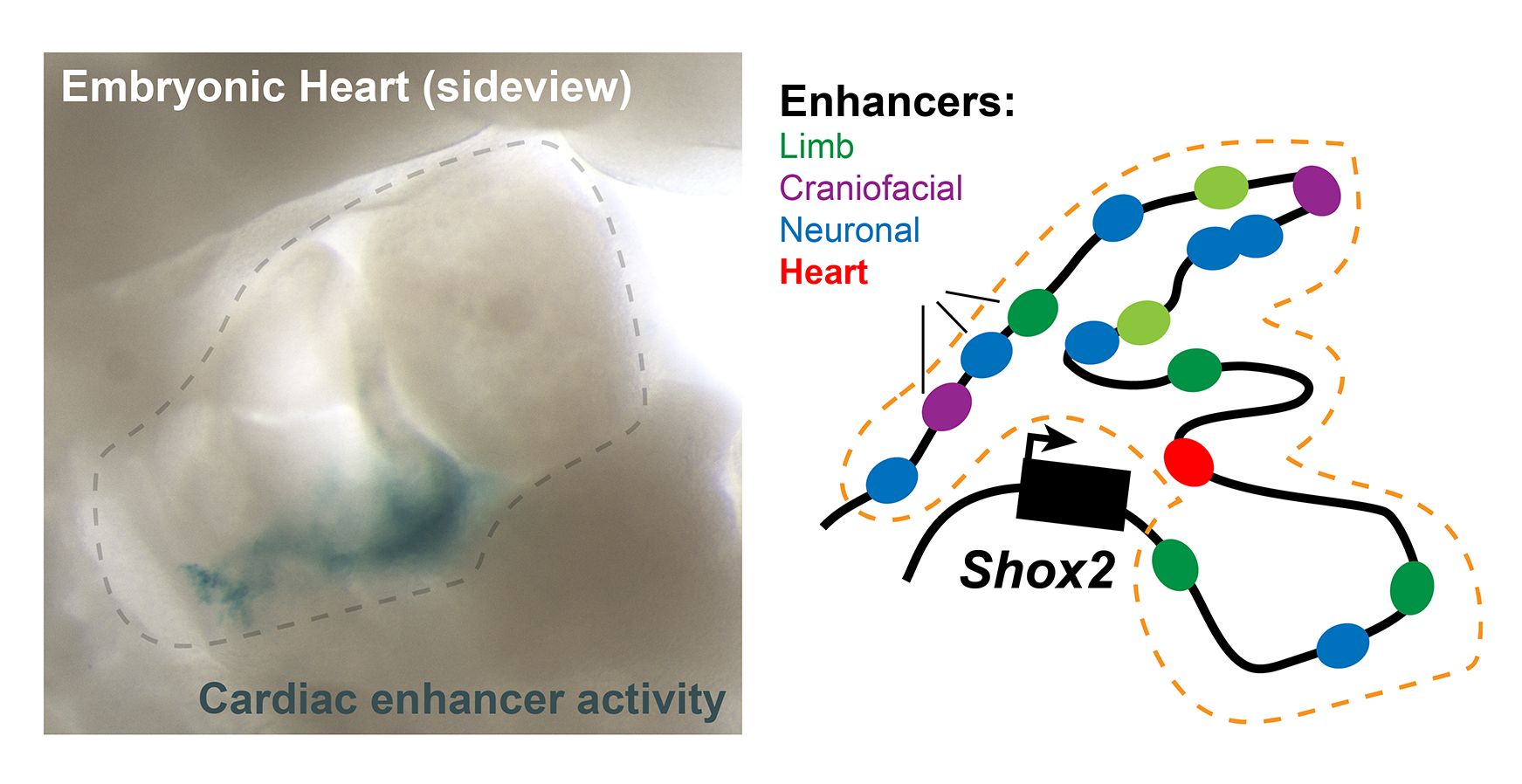
In a recent study supported by the Swiss National Science Foundation, an international research team led by Prof. Dr. Marco Osterwalder of the Department for BioMedical Research (DBMR) at the University of Bern and the Department of Cardiology at Inselspital, Bern University Hospital, used a mouse model to investigate the role of such a gene desert adjacent to the Shox2 gene. Shox2 is central to limb development and cardiac function in both humans and mice. In collaboration with Prof. Dr. John Cobb from the University of Calgary in Canada, the Lawrence Berkeley National Laboratory, USA, and other partners, the research team was able to show that the ‘deserted’ region flanking Shox2 contains important elements that regulate its activity. The team’s findings were recently published in Nature Communications.
Gene deserts contain key regulatory elements
Earlier research in mice had shown that cardiac disorders (e.g. arrhythmias) and even embryonic death may occur when Shox2 function is impaired. Mutations in the human variant of the gene have been associated with cardiac arrhythmias and the gene desert flanking the Shox2 gene shows a similar extension in humans and mice. How exactly the activity of the Shox2 gene is controlled in the embryo, however, remained unclear. Earlier studies had also shown that not all gene deserts are important for embryonic development.
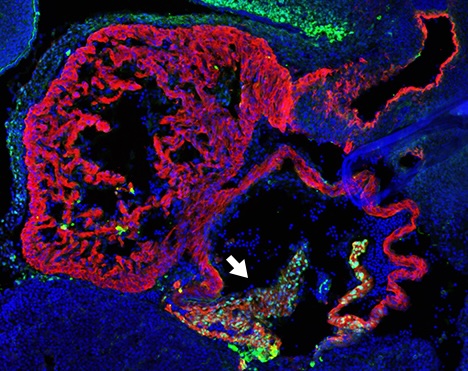
The recent study shows that the supposedly largely ‘empty’ gene desert DNA segment next to the developmentally important Shox2 gene does indeed contain a total of 15 regulatory elements, so-called ‘enhancers’. These enhancers control how and where the gene is active during embryonic development.
One example of the importance of these enhancers is their role in cardiac development. “In our study, we were able to demonstrate that a specific enhancer located in the gene desert co-regulates the activity of the Shox2 gene in developing heart cells in mice. This is particularly important because Shox2 plays a key role in the formation of the sinoatrial node, which acts as a natural pacemaker, by generating electrical impulses that control the heart rate,” explains Marco Osterwalder, co-corresponding author of the study.
Potential for genetic diagnostics
So far, there were only few examples of gene desert regions essential for the survival of mouse embryos and likely also for the survival of human embryos. “The present study not only identifies an important gene desert in the genome, but also shows how complex the mechanisms underlying the regulation of developmental genes are. These new findings can help us to better understand how enhancers work and how individual genes are active simultaneously in different cell types and tissues of the embryo,” says Osterwalder. The field of functional genomics and the study of diseases caused by defective enhancers are still relatively new areas of research and at the center of modern approaches to precision medicine. At the Faculty of Medicine of the University of Bern, these focal points are currently also being pursued as part of ‘PACE’, the ‘lighthouse’ project of the Bern Center for Precision Medicine (BCPM).
According to the researchers, the findings are particularly relevant for the ‘mapping’ of the human genome. They could also be of great importance for genetic diagnostics, especially in the context of modern personalized medicine. For example, genetic tests that detect mutations in gene deserts could in the future help to recognize the risk for birth defects or heart diseases such as arrhythmias at an early stage and allow them to be treated accordingly.
“As part of the Cardiovascular Disease (CVD) Programme at the DBMR and the Cardiovascular Research Cluster (CVRC) at the University of Bern and the Bern University Hospital (Inselspital), we will use the findings of our study to determine how the development of the sinoatrial node is anchored within the genome,” says Osterwalder. The researchers' aim is to identify all regulatory elements pertaining to the heart located within the analyzed gene desert and to investigate whether these could be affected by pathogenic mutations. “These findings could provide important contributions to genetic diagnostics and the treatment of cardiac arrhythmias,” concludes Osterwalder.
Publication details:Abassah-Oppong, S., Zoia, M., Mannion, B.J. et al. A gene desert required for regulatory control of pleiotropic Shox2 expression and embryonic survival. Nat Commun 15, 8793 (2024). https://doi.org/10.1038/s41467-024-53009-7 |
Department for BioMedical Research (DBMR)The Department for BioMedical Research (DBMR) of the Medical Faculty of the University of Bern was founded in 1994 by the University of Bern and the Inselspital, University Hospital Bern. The DBMR is divided into 13 research programmes with around 100 participating individual laboratories and several independent research laboratories whose research spans all biomedical areas. To bridge the gap between the laboratory and the bedside, the DBMR promotes clinical research with a strong emphasis on the development of translational approaches, the use of "omics" and other cutting-edge technologies, and extensive collaboration between laboratory-based and patient-centred clinical research. The DBMR is also committed to the promotion of young scientists. |
Cardiovascular Research ClusterThe Cardiovascular Research Cluster Bern (CVRC) was founded as a local network in 2015 to increase the visibility and awareness of ongoing cardiovascular research in Bern. It aims to foster collaboration between researchers and clinicians at the University of Bern and Inselspital, and to enrich the training environment for early career researchers as well as to provide a framework and logistical support for cross-team and/or interdisciplinary projects. The main goal is to strengthen Bern's position as a leading center for cardiovascular research on a national and international level, and to secure its competitive position for the future by promoting foundational, translational and clinical partnerships, enriching the training program for PhD students and increasing the visibility and attractiveness of Bern for researchers in the field of cardiovascular disease. The CVRC has built a strong community and strengthened the links between Bernese cardiovascular researchers. The CVRC now comprises over 400 members from 15 local institutes and departments. |
PACE - On the track of sudden cardiac arrestThe interdisciplinary project PACE (Precision Diagnosis and Therapy in CArdiac ChannelopathiEs) has been selected as a 'Lighthouse Project' of the Bern Center for Precision Medicine (BCPM) in 2023 and aims to develop a precise, personalized therapeutic approach to cardiac arrhythmias. The project is led by Prof. Dr. Katja Odening, Full Professor of Translational Cardiology, together with the other project partners, Prof. Nadia Mercader, Full Professor of Anatomy, Prof. Christiane Zweier, Full Professor of Human Genetics, Prof. Jean-Louis Reymond, Full Professor of Chemistry and Prof. Marco Osterwalder. The project was started at the beginning of 2024 at the University of Bern. In young people, an inherited ion channel disease is often associated with sudden cardiac arrest. As a first step, researchers are therefore focusing on identifying the disease-causing gene variants in patients' genetic material. They are also investigating the role of gene variants of unclear significance and genetic modifiers such as enhancers. To better understand the significance of these gene variants and modifiers, cell culture experiments are combined with model organisms (fruit flies, zebrafish, mice or rabbits), depending on the focus. Ultimately, this approach should provide insights into human disease mechanisms and new therapeutic approaches. Further information |
2024/11/06