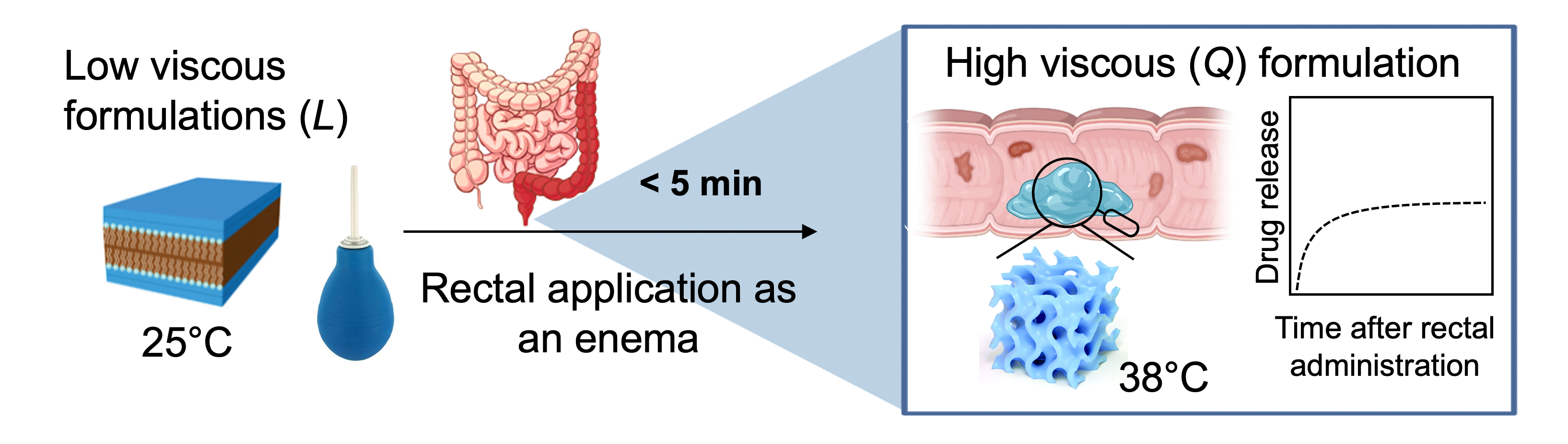
Treating the inflamed intestinal wall locally
Treatment of the chronic inflammatory bowel disease ulcerative colitis often produces unsatisfactory results. Researchers at the University of Bern have now developed a lipid gel that is administered directly to the inflamed part of the intestine, where it remains and releases its active substance evenly. This could result in a new, targeted therapy approach with fewer side effects.
For diseases that affect a specific organ or tissue, a drug is usually most effective and well-tolerated if it is administered exactly where it is supposed to work in the body. If it is swallowed or injected, it distributes throughout the body, thus increasing the risk of side effects.
Researchers from the Department of Chemistry, Biochemistry and Pharmaceutical Sciences and the Institute of Tissue Medicine and Pathology at the University of Bern, together with colleagues from the University Hospital Zurich, have developed a self-forming, viscous lipid gel to deliver anti-inflammatory drugs directly to the wall of the colon or rectum. Thanks to this innovation, patients with ulcerative colitis, a chronic inflammation of the terminal part of the intestine, could be helped in a more targeted way and with fewer side effects.
Existing therapies often unsatisfactory
Patients with ulcerative colitis suffer from cramping abdominal pain, diarrhoea, loss of appetite and weight, as well as fatigue. The aim of treatment is to make the inflammation disappear as completely as possible and for as long as possible. But often this does not work as desired. "For many patients suffering from ulcerative colitis, the side effects of an active principle taken orally can outweigh the therapeutic benefits," says Paola Luciani from the Department of Chemistry, Biochemistry and Pharmaceutical Sciences at the University of Bern. "Our gel, on the other hand, can hold a high amount of active ingredient and deliver it in a targeted manner."
Body heat thickens the gel
For their self-forming gel, the researchers chose a lipid that is well tolerated and safe for use in humans. It is a fluid material at room temperature and can be administered as an enema into the inflamed area of the colon. There, at body temperature, it forms a viscous and sticky gel and remains adherent for at least six hours, gradually releasing the active ingredient.
Acute intestinal inflammation treated in mice
There exists a very complex equilibrium in the intestinal environment. Numerous factors such as the intestinal wall, the immune system and microorganisms found in the intestine interact with each other. In the case of inflammation, this balance changes fundamentally. Therefore, after the first experiments with artificial membranes and intestinal tissue samples from rats, it was necessary to test the gel in living test organisms. For this purpose, the researchers used mice with an intestinal inflammation comparable to ulcerative colitis in humans and treated them with the gel over several days. The gel was loaded with one of two anti-inflammatory agents approved for the treatment of moderate to severe ulcerative colitis in humans. Both agents are taken orally and have significant side effects when taken orally.
A promising approach
"The novel delivery form showed very positive results," says Philippe Krebs from the Institute of Tissue Medicine and Pathology at the University of Bern. The health of the treated mice improved significantly. Compared to the control groups, they lost less weight, had better inflammation values and the signs of inflammation in the intestinal wall decreased more. Before the first trials with the self-forming drug depot in patients can follow, further tests on animal models are needed. Simone Aleandri from the Department of Chemistry, Biochemistry and Pharmacy at the University of Bern says: "We are confident that with our gel we can reduce the side effects of current therapies. The goal is a patient-friendly colitis therapy that relieves symptoms more effectively due to targeted and continuous drug delivery."
The study was financed by the Innovation Office of the University of Bern, the Swiss National Science Foundation, the Bern University Research Foundation and a "Seal of Excellence Fund" (SELF) of the University of Bern.
The research results were published in the journal Nature Communications.
Publication details:Marianna Carone, Marianne R. Spalinger, Robert A. Gaultney, Raffaele Mezzenga, Kristýna Hlavačková, Aart Mookhoek, Philippe Krebs, Gerhard Rogler, Paola Luciani, Simone Aleandri.Temperature-triggered in situ forming lipid mesophase gel for local treatment of ulcerative colitis. Nat Commun. 2023 Jun 13;14(1):3489. https://doi.org/10.1038/s41467-023-39013-3 |
Department of Chemistry, Biochemistry and Pharmaceutical SciencesResearch at the Department of Chemistry, Biochemistry and Pharmaceutical Sciences (DCBP) is thematically divided into two areas: (I) Chemistry and Biochemistry, (II) Pharmacy. At the Department, more than 20 university lecturers teach and conduct research and modern education is offered with Bachelor's and Master's programmes in three different fields of study: "Chemistry and Molecular Sciences", "Biochemistry and Molecular Biology" and "Pharmaceutical Sciences", with a subsequent doctoral programme in the fields of chemistry and biochemistry. |
Institute of Tissue Medicine and PathologyThe Institute of Tissue Medicine and Pathology (IGMP) at the University of Bern covers the entire range of morphological and molecular diagnostics on tissue samples. The combination of service, teaching and research under one roof allows close interaction and mutual inspiration. Research is concerned with the development, diagnosis and therapy of diseases. Immunopathologies, inflammatory diseases and aspects of tumour biology form the current thematic focus. Ex-vivo investigations are carried out on human tissue samples and experimental in-vitro and in-vivo model systems are also used. As a university center for tissue medicine and pathology, the IGMP offers the entire spectrum of tissue examinations. Contact partners are defined for each medical field, who are well networked in the corresponding interdisciplinary environment. |
2023/10/24